The WW-domain scaffold for the de novo design of miniaturized phosphate receptors, phosphatases and sulfatasesResearch - Christina Lindner |
|
|
|
|
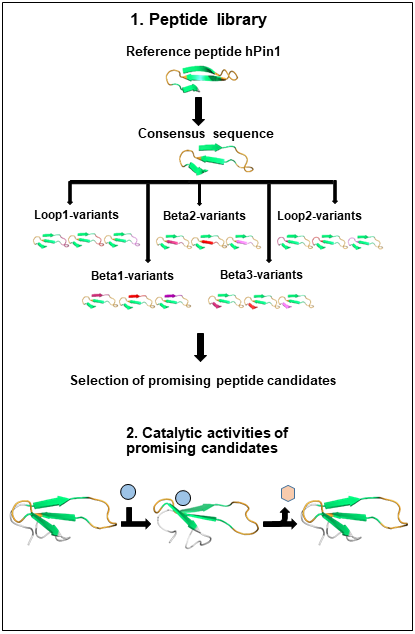
A major goal of research in the field of synthetic chemistry is the design and production of peptide- and protein structures that function as biological synthesis machines. At the beginning of de novo design of peptide catalysts the focus was set on helix bundles as potential scaffold-material. Based on the fact that these are relatively rigid and self-assembling scaffolds, current scientific efforts are on smaller β-sheet motifs such as the SH3- or the WW-domain. In my project the WW domain was selected as potential scaffold for the design of miniatured enzymes due to its properties as a small independently folding protein motif. It is a protein interaction module with 34-40 amino acid residues and has a flexible binding site. The main focus of this research project is the design of a universal and stable WW-domain similar β-sheet scaffold, based on which a molecular recognition site can be constructed. Besides of binding properties to different binding motives the construction of a recognition site of phosphorylated and sulfated peptides and molecules is planned. This should lead to the generation of WW-domains with phosphatase and sulfatase properties. Based on the sequence comparison of 85 WW-domains a consensus sequence was identified that serves as a starting point for the de novo design of a WW-domain scaffold. In the following the design for β-strand sequence segments and then for loop regions will be tested. Based on these results a stable folding WW-domain scaffold will be generated. Suitable candidates will be investigated concerning their structure using CD, IR and NMR-spectroscopy. Another central project part is the development of efficient binding assays such as FRET-based binding studiesst. This PhD project will make an important contribution for the design of small catalysts based on protein folding motives, which are especially relevant for the field of synthetic biology and chemistry. |

